The Offenbacher research program is centered on the following areas of protein biochemistry and biophysics.
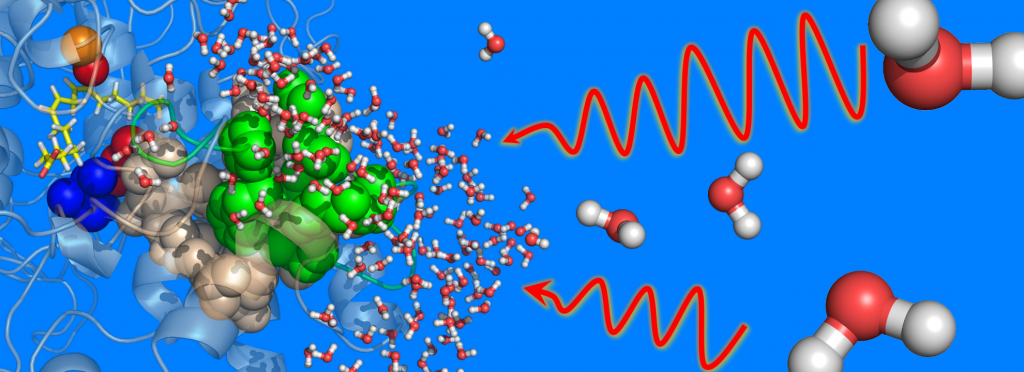
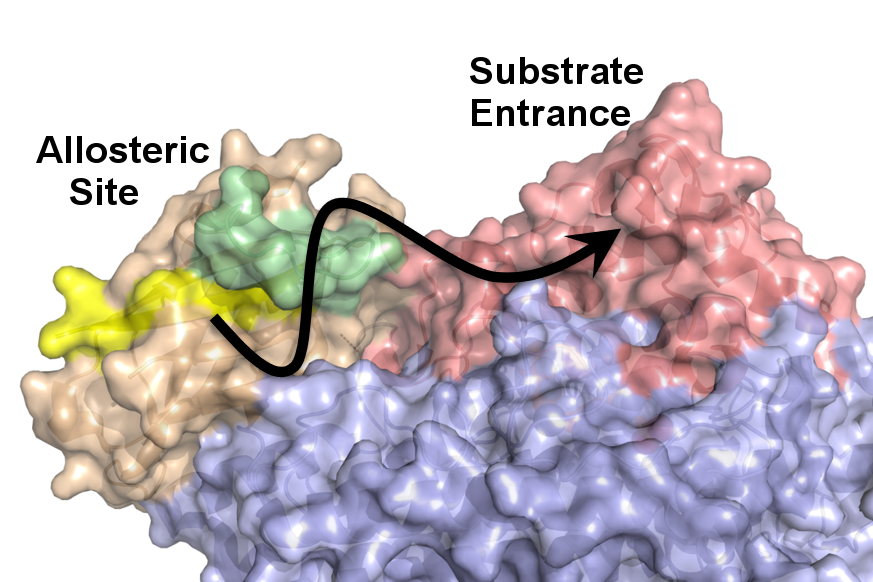
Protein Dynamic Allostery of Plant and Animal Lipoxygenases
A number of enzymatic processes important for sustaining life are highly regulated and often activated by long-range dynamic processes, known as allostery. These processes are typically initiated by binding a small biologically relevant molecule at one site on the protein matrix triggering a shift in the ensemble of conformational (sub)states. Allosteric effects can be found remote from and transmitted (dynamically) to the active site where chemistry takes place.
Lipoxygenases (LOXs) produce/generate a diverse set of oxidized (primarily hydroperoxides) products, through the C-H activation of polyunsaturated fatty acids and phospholipids forming potent, bioactive lipid mediators. In humans, these products lead to either anti- or pro-inflammatory effects. There are six isoforms of human LOXs with diverse functional roles; only one has been targeted by an FDA-approved anti-inflammatory (Zileuton).
Our lab utilizes a combination of isothermal calorimetry (ITC), X-ray crystallography, and the innovative application of temperature-dependent hydrogen deuterium exchange (TD-HDX) methodologies that provide a unique window into the structure and dynamics of protein-effector interactions that tightly regulate LOXs structure and function. Such information has the potential to define allosteric effector interaction sites to be leveraged for development of novel anti-inflammatories for the treatment of chronic inflammation. We are also investigating the structural and functional consequences of the interactions of the LOX protein at the membrane interface. Besides the practical value, this project will provide important fundamental insight into the long-range dynamic allosteric regulation of substrate selectivity and reactivity in biological catalysis – a long-standing challenge in modern biochemistry.
We are currently collaborating with Prof. Theodore Holman (University of California, Santa Cruz; drug design) and Prof. Marcia Newcomer and Dr. Nathan Gilbert (LSU; X-ray crystallography) on this project. This work is supported by (in part) an NIH grant.
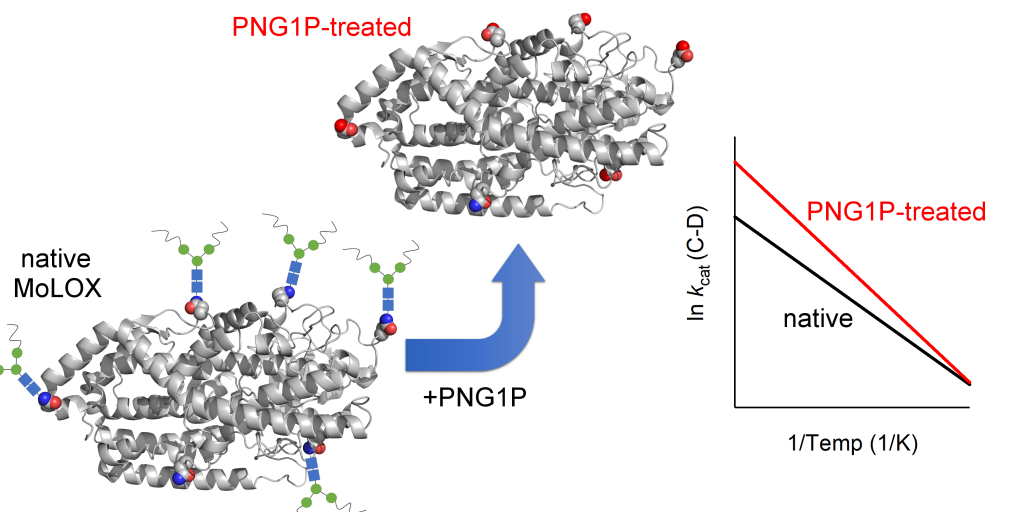
Structure-Function Analysis of Manganese Lipoxygenases from Pathogenic Fungi
Lipoxygenases from pathogenic fungi belong to the lipoxygenase family that catalyze C-H activation of polyunsaturated fatty acids to form a diverse set hydroperoxidizes. While the lipoxygenase catalytic domains are structurally and functionally similar, lipoxygenases originating from pathogenic fungi are quite unique from the canonical enzymes found in plants and animals in several regards, including: i) a functionally substituted manganese reactive center, ii) formation of a non-conjugated hydroperoxide products, and iii) presence of heterogenous, N-linked glycans. One exemplary system is MoLOX, a lipoxygenase from the fungus M. oryzae, that is emerging as an important target for the devastating rice blast disease. Our lab is focused on the structural features that give rise to these functional differences. We have recently shown that the hydrogen transfer, associated with the C-H cleavage of the substrate linoleic acid by MoLOX, is rate-limiting and occurs by a hydrogen tunneling mechanism. Further, the differential enthalpic barrier for H and D transfer, ΔEa, has served as a kinetic reporter of tunneling efficiency, and showing that loss of N-linked carbohydrates is linked to a disproportionate increase in the activation energy for deuterium transfer. These data are consistent with an impairment of both the tunneling ready state and the ground state enzyme-substrate structure. Our lab is now focused on resolving the structural role of N-linked glycosylation through a combination of structural techniques, including X-ray crystallography, HDX-MS, and a high precision electron-nuclear double resonance (ENDOR) strategy. We are currently collaborating with Profs. Brian Hoffman (Northwestern) and Martin St. Maurice (Marquette). The emerging data have important implications towards MoLOX inhibitor design.
Resolving the Structural Differences between Fibrinogen and Fibrin to Provide Novel Therapeutic Targets
Fibrinogen plays a key role in blood clot formation that is essential to wound healing , but is also linked to several human blood related diseases, including stroke. Human fibrinogen is a soluble, 340 kDa glycoprotein and is the third most prevalent protein found in blood plasma. Despite a wealth of structural information on fibrin fibers (polymerized form), we still do not have a clear understanding of the molecular mechanisms for the processing and mechanism of polymerization. Quite surprisingly is the lack of a complete structure for the soluble, monomeric form. Our team is working with Profs. Nathan Hudson at ECU and Mehmet Sen at University of Houston to resolve the structure of fibrinogen and the impact of the protein structure on the glycan architecture. The latter is anticipated to be influenced during pregnancy and could play an important role in pregnancy related complications. Further, we are working to shed light onto the mechanism for the transition of soluble monomeric fibrinogen to fibrin, the polymerized form.
This work is supported by the NIH.
